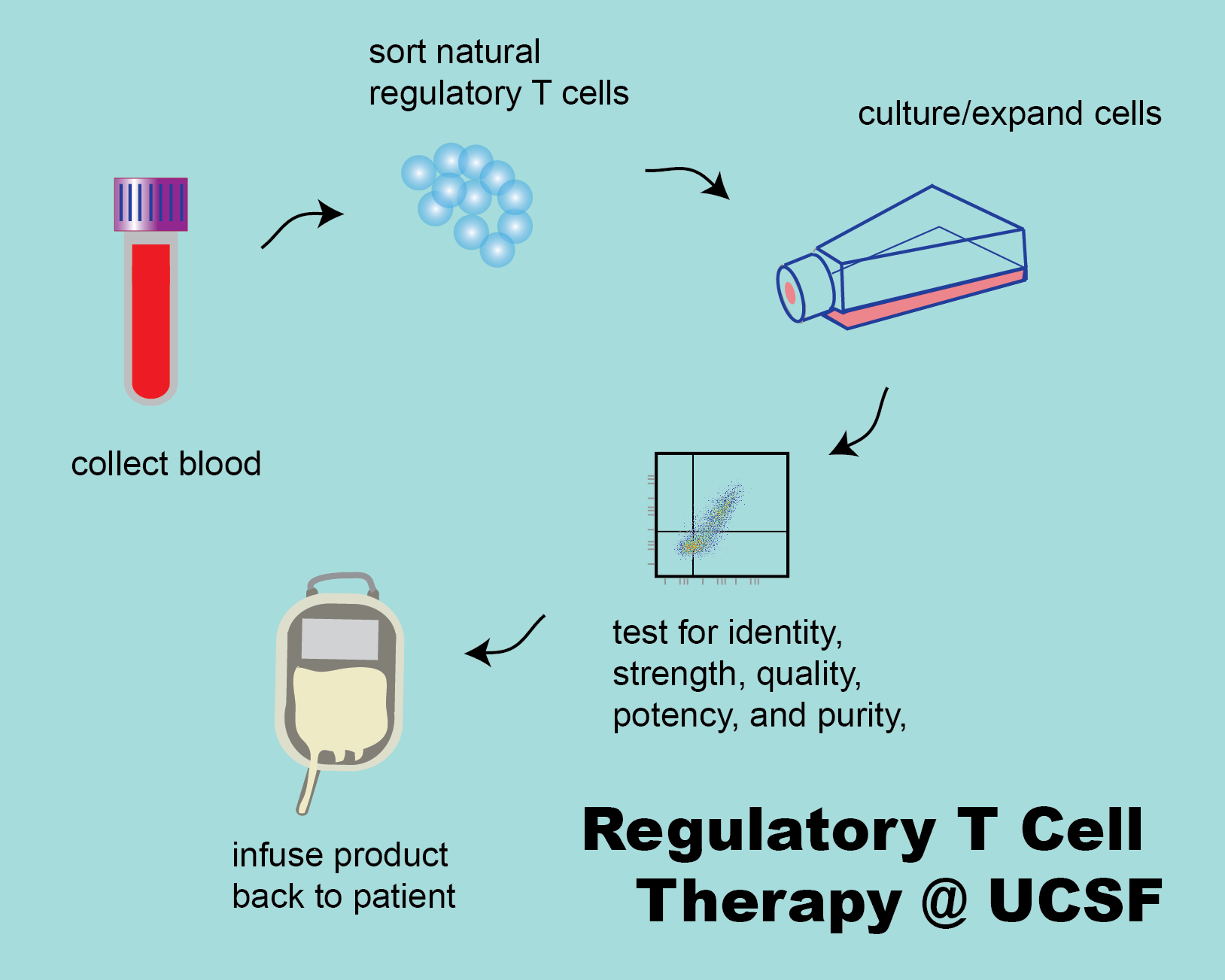
Regulatory T cells (Tregs) are a subset of CD4+ T cells that have anti-inflammatory properties. In animal models, they have the ability to prevent rejection of allografts (tissue from another, genetically distinct animal) and to ameliorate the symtoms of autoimmune disease.
Our group manufactures Ex Vivo Expanded CD4+CD127lo/-CD25+ Alloantigen- Reactive Regulatory T Cells (arTregs) and polyclonal CD4+CD127lo/‐CD25+ regulatory T cells (polyclonal Tregs) for clinical trials in patients who have received solid organ tranplant or who have been diagnosed with an autoimmune disease. The goal is to test the safety and efficacy of these cells to prevent solid organ rejection or to improve symptoms of autoimmune disease.
These cellular therapy products are investigational new drugs limited by federal law for investigational use only. Polyclonal Tregs and arTregs are manufactured at the Human Islet and Cellular Transplantation Facility (HICTF) and GMP Facility, an FDA registered facility located at the University of California, San Francisco (UCSF).
Publications from the regulatory T cell program
Polyclonal Regulatory T Cell Therapy for Control of Inflammation in Kidney Transplants. Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, Leung J, Nguyen V, Sigdel T, Tavares EC, Yang JYC, Hellerstein M, Fitch M, Bluestone JA, Vincenti F. Am J Transplant. 2017 Jul 4. doi: 10.1111/ajt.14415.
Transplant trials with Tregs: perils and promises. Tang Q, Vincenti F. J Clin Invest. 2017 Jun 30;127(7):2505-2512. doi: 10.1172/JCI90598.
Inducing and Administering Tregs to Treat Human Disease. Perdigoto AL, Chatenoud L, Bluestone JA, Herold KC. Front Immunol. 2016 Jan 22;6:654. doi: 10.3389/fimmu.2015.00654.
Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Sci Transl Med. 2015 Nov 25;7(315):315ra189. doi: 10.1126/scitranslmed.aad4134.
Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, Trotta E, Szot GL, Liu W, Lares A, Lee K, Laing A, Lechler RI, Riley JL, Bluestone JA, Lombardi G, Tang Q. Am J Transplant. 2013 Nov;13(11):3010-20. doi: 10.1111/ajt.12433.
